In an era where technology shapes much of our healthcare experience, the importance of Equity-Centered Design (ECD) in federal Health IT initiatives cannot be overstated. This approach is pivotal in ensuring that health IT systems are not just efficient and user-friendly, but also equitable and inclusive for all users.
What is Equity-Centered Design?
Equity-Centered Design is an approach that integrates the principles of equity and inclusivity into the design process. Unlike traditional design methodologies that often cater to the majority, ECD specifically addresses the needs of marginalized and underrepresented communities in healthcare. It aims to identify and dismantle barriers to access and usage in health IT systems, ensuring that these technologies serve everyone equitably.
Focusing beyond technology and user-centered approaches toward human-centered and ECD requires meaningful contextual investigation and the engagement of broader stakeholders and communities, as shown in Figure 11. While technology and user-centered approaches can facilitate the usability and feasibility of an innovation, human and equity-centered approaches can also increase its acceptability and adoption, ultimately having a greater impact on health equity.
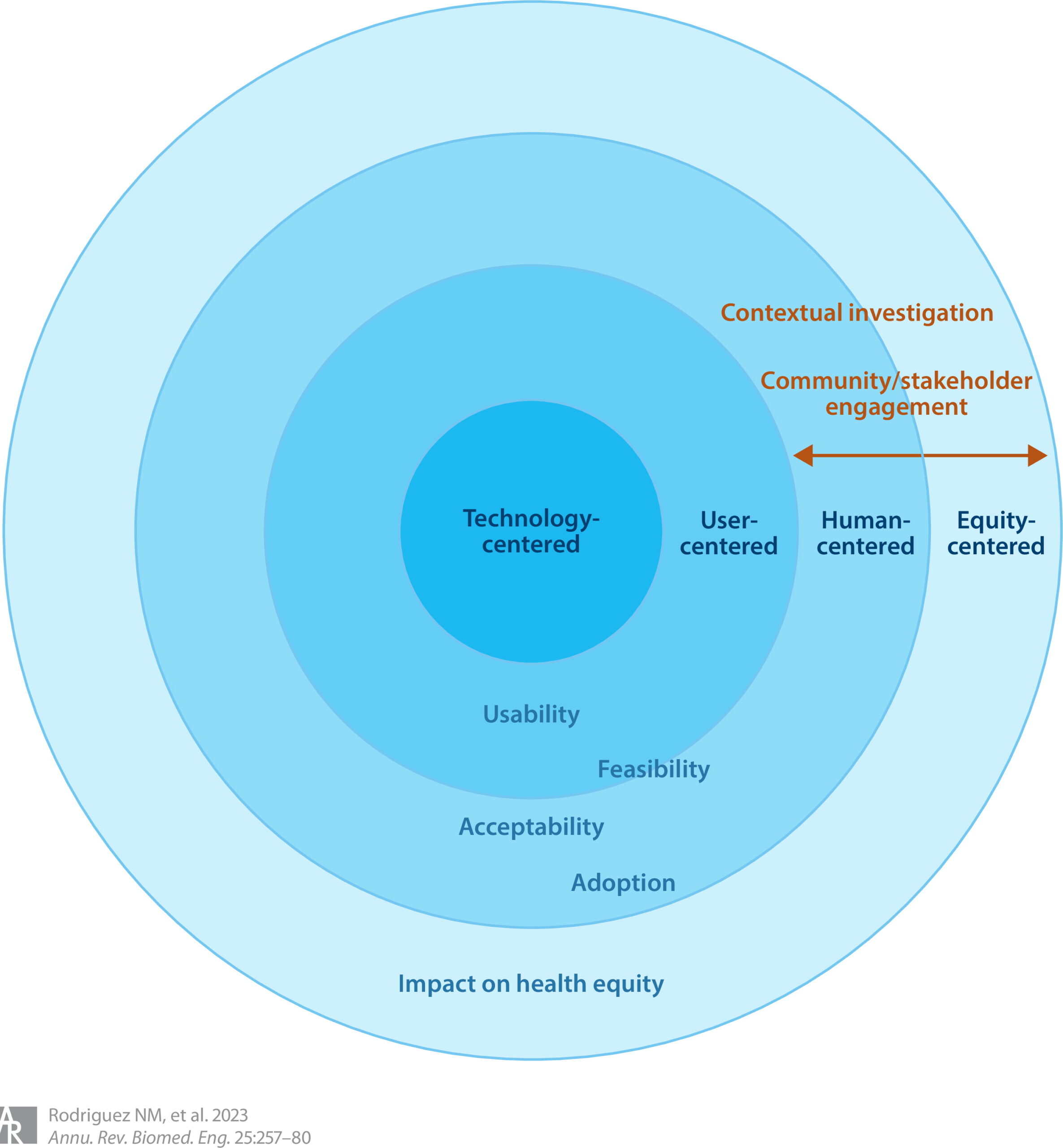
Figure 1
How Does Equity-Centered Design Work?
Implementing ECD involves the following steps:
- Research and Understanding: Begin by researching and understanding the diverse needs of the target user groups, especially focusing on those who are often overlooked.
- Inclusive Ideation: Involve stakeholders from diverse backgrounds in the ideation process to ensure that a wide range of perspectives is considered.
- Design and Development: Develop solutions that are not only functional, but also accessible and sensitive to the unique needs of different user groups.
- Testing and Feedback: Continuously test the system with a diverse user base and incorporate feedback to make iterative improvements.
Case Study: Implementing ECD in a Health IT Project2
Cornet et al. designed an application for older adults with heart failure and integrated user expertise across the formative research, design, and evaluation stages of their work. At the formative stage, patient interviews, advisory meetings, and meetings with clinician advisors were incorporated to establish the problem scope and design requirements to support older adults with a cardiac implantable electronic device. At the development stage, designers generated prototype user-interface dashboards and worked with user participants to determine the preferred information flow and layout. Three groups of three individuals, consisting of a mix of elderly patients and informal caregivers, provided feedback at the prototype stage of the design. Lastly, usability and heuristic evaluations with patients and user-centered design experts informed final development stages.
Takeaway: Throughout the process, the designers proactively sought innovation equilibrium. They achieved this by:
- Involving diverse and representative stakeholders early on
- Managing designer assumptions through validation and feedback with stakeholders
- Managing stakeholder differences
- Accommodating clinical workflows and regulatory limitations
- Balancing the desire to create overly complex designs for the sake of innovation with the practical user needs for simplicity
Conclusion
The integration of ECD in federal Health IT initiatives is more than a trend; it’s a necessary evolution in how we approach healthcare technology. By focusing on equity, we can ensure that these technologies provide meaningful benefits to all, particularly those who have been historically underserved by healthcare systems.
It’s time for all stakeholders in the federal Health IT space to embrace ECD. Whether you’re a developer, policymaker, or healthcare provider, your commitment to equity can lead to profound and positive changes in our healthcare system.
Source:
Thinking Beyond the Device: An Overview of Human- and Equity-Centered Approaches for Health Technology Design –
Natalia M. Rodriguez, Grace Burleson, Jacqueline C. Linnes, Kathleen H. Sienko
Annual Review of Biomedical Engineering 2023 25:1, 257-280
1. https://www.annualreviews.org/doi/full/10.1146/annurev-bioeng-081922-024834#_i8
2. Section 3.2.1 from https://www.annualreviews.org/doi/full/10.1146/annurev-bioeng-081922-024834#_i8






